Venus Medtech VenusP-Valve Completed First Implantation in IDE Pivotal Clinical Study in U.S.
HANGZHOU, China, June 14, 2024 /PRNewswire/ -- Venus Medtech (02500. HK), a leading provider of integrated solutions for transcatheter structural heart valvular therapies in China, announced today that its in-house developed innovative transcatheter pulmonic valve replacement (TPVR) system, VenusP-Valve, has completed its first implantation in the PROTEUS IDE Pivotal Clinical Study at the University of Virginia School of Medicine. This momentous procedure was performed by a multidisciplinary team coordinated by Prof. Scott Lim and Prof. Michael Hainstock of the center.
This marks a significant advancement in the international progress of VenusP-Valve and represents another milestone following its CE MDR approval in April 2022.
The VenusP-Valve PROTEUS STUDY, abbreviated from Evaluation of the PerfoRmance Of The VEnUsP-Valve System in Patients with Native Right Ventricular Outflow Tract (RVOT) Dysfunction, is a prospective multi-center non-randomized interventional study in patients with RVOT disorders comorbid with moderate or greater pulmonary regurgitation. With a target enrollment of 60 subjects, data from this trial will support VenusP-Valve's registration with the U.S. FDA and Japanese Pharmaceuticals and Medical Devices Agency (PMDA).
In late 2023, the VenusP-Valve PROTEUS trial received coverage approval from the U.S. Centers for Medicare & Medicaid Services (CMS). With this endorsement, all eligible beneficiaries can be reimbursed for VenusP-Valve treatment in the study.
Following its first clinical implantation in 2013, VenusP-Valve has been applied in clinical practice for 11 years. To date, the device has been included in national health insurance programs in Germany, France, etc., and has been approved in more than fifty countries, including China, Germany, France, the United Kingdom, Italy, Spain, Canada, and Australia, with its implantation seeing continuous growth in new hospitals and centers.
"We're honored to be part of the VenusP-Valve PROTEUS trial," said Prof. Scott Lim after the procedure. "We've just completed the first patient enrollment in that trial using the VenusP-Valve. This worked quite well, and it really represents a possibility of better ways of treating patients with significant pulmonary valve disease."
"That went really smoothly in this teenage patient with free pulmonary insufficiency", commented Prof. Michael Hainstock. "I'm very happy with the valve deployment and position. This (VenusP-Valve) is another option for our patients to treat pulmonary valve disease."
"The successful first implantation in the VenusP-Valve PROTEUS study in the U.S. represents an important milestone for Venus Medtech in this crucial market," said Lim Hou-Sen, General Manager and CEO of Venus Medtech. "The device has already received compassionate use approval from the FDA in a number of cases, underscoring its unique clinical advantages and high regulatory recognition. Moving forward, we will redouble efforts to advance VenusP-Valve's clinical progress, speeding up its approval process with both the FDA and Japan's PMDA."
About VenusP-Valve
As the first self-expanding TPVR product approved in China and Europe, VenusP-Valve carries remarkable clinical value. Uniquely designed with both flared ends, the product ensures the blood flow of branchial artery with bare stents at the outflow end. It provides a stable multi-point anchoring system and enables easy delivery, with no need for pre-stenting before the procedure. Available in a variety of specifications with extensive applicability, VenusP-Valve is able to meet the needs of 85% of patients in the case of large RVOT.
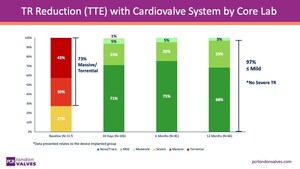
The long-term safety and efficacy of VenusP-Valve has been backed by impressive clinical data. According to three-year follow-up results of the clinical trial in Europe, the product demonstrated 100% procedural success and 0% all-cause mortality and reoperation among 81 patients who underwent TPVR. Right ventricular function improved significantly. Only one patient had severe pulmonary regurgitation.
About Venus Medtech
Venus Medtech (Hangzhou) Inc. (02500.HK) is committed to structural heart innovation. We are developing and commercializing comprehensive solutions for structural heart disease. Our robust pipeline, encompassing all four heart valves from TAVR, TPVR, TMVR, and TTVR to hypertensive renal denervation (RDN) therapy, underscores our unwavering commitment.
For more information, please visit https://www.venusmedtech.com
*Provided for informational and academic purposes only, this content is not intended as professional medical or legal advice. Venus Medtech makes no representations, warranties or guarantees regarding the completeness, accuracy, or timeliness of this content. |
*Venus Medtech makes no representations, warranties or guarantees regarding the property or clinical performance of any medical devices mentioned. |
*VENUSMEDTECH, the stylized QI logo, VenusP-Valve, etc. are trademarks of Venus Medtech (Hangzhou) Inc. |
Copyright 2024. Venus Medtech (Hangzhou) Inc. All Rights Reserved. |
Contacts:
Jill Liu
Public Relations
liujie@venusmedtech.com
Ophelia Chen
Investor Relations
chenwenjuan@venusmedtech.com
Photo - https://mma.prnewswire.com/media/2438670/image_821354_23322949.jpg
Photo - https://mma.prnewswire.com/media/2438671/image_821354_23323838.jpg





Share this article