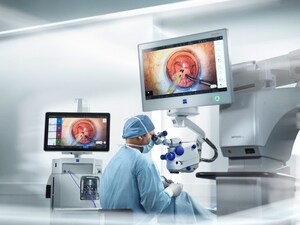
JENA, Germany, March 27, 2025 /PRNewswire/ -- ZEISS Medical Technology announced today that the ILM staining dye ILM-Blue® from DORC (Dutch Ophthalmic Research Center (International) B.V.) has received National Medical Products Administration (NMPA) approval in China, marking a significant milestone in the product's global journey.
"ZEISS and DORC are committed to supporting surgeons in the China market with the latest innovation and tools that create a higher standard of care and better patient experience," says Pierre Billardon, Head of Business Sectors Surgery Posterior Segment at ZEISS Medical Technology and CEO of DORC International. "The global success of ILM-Blue underscores the company's commitment to providing high-quality and effective solutions to the ophthalmic community."
"We are delighted to bring ILM-Blue to the Chinese market, where it will provide retina surgeons with a proven, high-quality staining solution to enhance visualization," says Jessie Jiang Bo, General Manager of DORC in China. "This approval reinforces our dedication to expanding access to innovative ophthalmic solutions that help surgeons to improve patient outcomes."
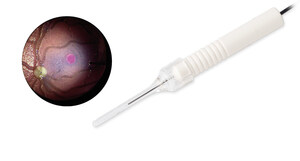
ILM-Blue® is used to clearly stain and easily distinguish the inner limiting membrane (ILM) from underlying retinal layers during vitreoretinal surgery. This facilitates membrane removal and reduces the risk of retinal damage1. Since its launch in 2010, ILM-Blue® from DORC has been used in more than 900,000 procedures worldwide2, consistently supporting vitreoretinal surgeons to improve patient outcomes and setting new standards in posterior segment surgery. With this latest approval, ILM-Blue® becomes the first DORC posterior dye product available in China, bringing its trusted solution and proven benefits3,4 to healthcare professionals and patients in the region.
To date, ILM-Blue® remains the only ILM staining solution approved by the FDA. It is marketed under the brand name TissueBlue® in the U.S. and is listed as the #1 preferred ILM stain among U.S. retina surgeons5.
1 Instructions for use.
2 DORC data on file.
3 Figueroa M.S. et al; Long-term outcomes of 23-gauge pars plana vitrectomy with Internal Limiting Membrane peeling and gas tamponade for myopic traction maculopathy, retina: September 2015 - volume 35 - issue 9 - p 1836-1843 doi: 10.1097/iae.0000000000000554.
4 Veckeneer, M., Mohr, A., Alharthi, E., Azad, R., Bashshur, Z. F., Bertelli, E., ... & Melles, G. R. (2014). Novel 'heavy'dyes for retinal membrane staining during macular surgery: multicenter clinical assessment. Acta Ophthalmologica, 92(4), 339-344.
5 2023 survey of US retinal Surgeons, ASRS meeting, Seattle.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates do not have clinical evidence supporting the opinions and statements of the healthcare professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support.
Contact for investors
Sebastian Frericks
Head of Group Finance & Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220-116
Email: investors.med@zeiss.com
Contact for the press
Frank Smith
Head of Global Communications Ophthalmology
Carl Zeiss Meditec Inc.
Phone: +49 3641 220 331
Email: press.med@zeiss.com
About Carl Zeiss Meditec AG
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With 5,730 employees worldwide, the Group generated revenue of €2,066.1m in fiscal year 2023/24 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 39 percent of Carl Zeiss Meditec AG's shares are in free float. Approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries.
For more information visit our website at www.zeiss.com/med
About D.O.R.C. Dutch Ophthalmic Research Center (International) B.V.
D.O.R.C. is one of the world's leading suppliers of equipment, instruments and liquids for ophthalmic surgery. For over 40 years, D.O.R.C. has grown into a successful international business, shaping its product portfolio through close collaboration with leading top surgeons. The company improves eye surgery globally and maximizes surgeon control by providing innovative quality approaches for eye disorders. Its products are exported to more than 80 countries worldwide. The company is headquartered in Zuidland, the Netherlands, and has more than 800 employees. In April 2024, D.O.R.C. was acquired by Carl Zeiss Meditec AG.
Photo - https://mma.prnewswire.com/media/2650992/ILM_BLUE.jpg
Logo - https://mma.prnewswire.com/media/546786/ZEISS_v1_Logo.jpg





Share this article